In today's “Li-ion Battery 101” blog post we'll talk about "Li-ion vs. Other Chemistries."
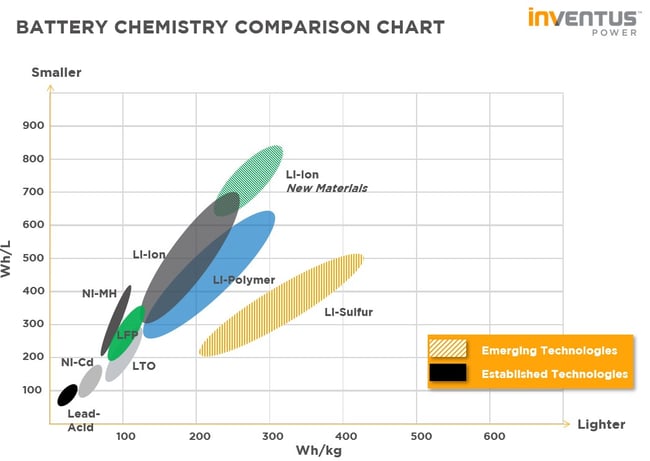
In the world of rechargeable batteries, there are only a few different cell chemistries that have seen widespread adoption over the years. Within the last few decades, Lithium-ion has become a preferred choice due to its inherent strengths over the other chemistries. But what are these strengths and why should one choose Li-ion for their application? We’ll review some of the most common rechargeable cell chemistries to understand their advantages and disadvantages and illustrate why Li-ion is leading the way in battery technology.
Lead Acid
If you ever opened the hood of a car, you've probably seen a Lead Acid (PbAc) battery. Lead Acid batteries have been around since the mid-1800s and have been the power source for applications such as lawn and garden equipment, backup power supplies (UPS), automotive vehicles, and more. There is a variety of Lead Acid (PbAc) cell types such as:

- VLA (Vented Lead Acid), also called Flooded or Wet, or Maintenance-Free
- VRLA (Valve-Regulated Lead-Acid), sometimes referred to as SLA (Sealed Lead-Acid), Gel Cell, or Hybrid
- VRLA /AGM (Absorbed Glass Mat)
- VRLA/TPPL (Thin-Plate-Pure-Lead) and VRLA /Gel
The Lead Acid (PbAc) cell chemistry is a seasoned technology and batteries that use this chemistry have their advantages. Although Lead Acid batteries are very popular, due to their ability to produce high currents and low upfront cost, there are some disadvantages. Lead Acid (PbAc) batteries are large, heavy, slow charging, tend to have a shorter life span, and are not the most environmentally friendly compared to other batteries.
NiCad
The Nickel Cadmium (NiCad) cell chemistry came out in the late 1890s /early 1900s to compete with Lead Acid cells. By offering higher energy density, faster charge time, and longer cycle life, it became the choice in applications like power tools, mobile phones, laptops, flash lights, toys, and some stationary medical equipment. Compared to the Lead Acid, NiCad cells had some disadvantages like memory effect, high self-discharge, and heavier weight. Additionally, it contains Cadmium, a non-environmentally friendly toxic material that is banned in most countries.
NiMH
Another battery cell chemistry that's been used just about everywhere is Nickel-Metal Hydride (NiMH or Ni–MH). Since the late 1980s, this battery cell has powered devices from video cameras and electric toothbrushes, to large home appliances and medical equipment. Compared to their predecessor Nickel–Cadmium (NiCad), NiMH cells were developed for high capacity, rapid charging, high performance, and long life. There are some disadvantages of NiMH such as high self-discharge, weight, higher internal resistance, some memory effect, and less cycle life than NiCad. NiMH cells also need longer and special charging control to minimize heating and forming hydrogen gas.
Li-ion
The chemistry of choice at Inventus Power is Lithium-ion (Li-ion). Li-ion cells emerged in the battery industry in the early 1990s and remain highly popular today for their high energy density by volume and weight, high cycle life, relatively low self-discharge, and low maintenance. Cell phones, laptops, electric cars, and medical devices like heart pumps, are just some of the many products that are powered by Li-ion cells.
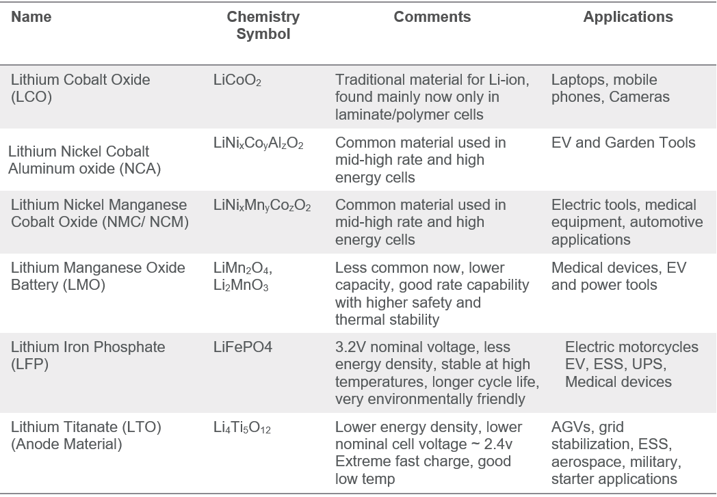
When we refer to Lithium-ion batteries, there is a multitude of Li-ion “flavors” or variants that have been created over the years. These variants are derived from using different anode/cathode materials. Below is a list of common Lithium-ion chemistries, LCO, NCA, NCM, LFP are various cathode materials. LTO is alternative anode material to graphite/carbon.
Li-ion has some distinct advantages in the marketplace, but this chemistry does require some attention due to its makeup. All lithium-ion battery packs require, at a minimum, a protection circuit, but often requires a full Battery Management System (BMS), to mitigate any potential risk of an unsafe chemical reaction taking place within the electrolyte. When Li-ion cells are manufactured safely and battery packs are designed with these high-quality cells and a proper BMS, safety risk is extremely low. You will learn more about Li-ion battery safety in an upcoming blog post.

Now that you have learned about the advantages and disadvantages of various cell chemistries, in our next Li-ion Battery 101 blog post, we’ll discuss the different cell types that are available for Li-ion batteries.
Written by: Imad Idelah, Principal Applications Engineer, Inventus Power
